Determination of prothrombin activity in plasma with Chromogenic Substrate S-2238™
Background
A number of studies during the last few years support the notion that venous thromboembolism (VTE) is a multifactorial disease most often triggered by circumstantial risk factors (trauma, surgery, pregnancy, oral contraceptives, immobilization and age) in combination with one or more genetic or acquired coagulation disorders (see ref. 1 of a review).
Elevated activity of prothrombin in the absence of a known underlying genetic disorder is also associated with an increased thrombotic risk (2). A mutation G to A in the untranslated 3’-region of the prothrombin gene at nucleotide position 20210 constitutes a risk factor for VTE with an odds ratio of 3-5 (2-10). About 90% of the carriers of this mutation have elevated levels (>115%) of prothrombin activity (2,7,8) .
Levels above the upper limit of the normal range (75 – 130%) are commonly hetero- and homozygotes (2,7-9). So far, there is no explanation why a comparatively mild increase of prothrombin activity constitutes a risk factor for thrombosis and this is therefore an area of active clinical and biochemical research. Chromogenic methods for accurate determination of elevated activities of prothrombin and other coagulation factors, such as factor VIII (11,12) are important tools for assessing the risk for VTE in patients and family members.
Measurement Principle
Prothrombin is activated to meizothrombin by the snake venom enzyme Ecarin from Echis Carinatus. After a certain incubation time, the amount of meizothrombin formed is measured with the thrombin selective substrate S-2238, which also is cleaved by meizothrombin. The absorbance recorded at 405 nm is proportional to the prothrombin activity in the sample.
Ecarin
Prothrombin → Meizothrombin
Meizothrombin
S-2238 → pNA + Peptide
Reagents
- Tris BSA Buffer
Buffer for sample dilution, containing 0.5 mol/l Tris HCl pH 7.3, I= 2.0 with NaCl and 2% bovine serum albumin. Before use, dilute the stock solution 1+9 with sterile water to obtain a buffer working solution. The buffer working solution is prepared and used within the same day. - Prothrombin Activator Diluent
Buffer for dilution of Ecarin, containing 0.05 mol/l Tris HCl pH 7.6, I=0.15 with NaCl, bovine serum albumin, polyethylene glycol and a fibrin polymerization inhibitor. - Ecarin
Reconstitute with sterile water according to the Ecarin package insert. Freeze in suitable aliquots at -20°C or at -70°C. Stable for 3 months at both storage temperatures. Before use, dilute with Prothrombin Activator Diluent to obtain a concentration of 0.6 U/ml. Stable for 8 hours at 20-25°C and for 1 week at 2-8°C. Note: Echis Carinatus crude venom can also be used. A suitable final concentration of this reagent is approximately 5 µg/ml; however, this may vary between different sources. 10-20% loss of activity may occur upon freezing at -20°C. - Chromogenic Substrate S-2238, Art. No. S820324
Reconstitute with 13 ml of sterile water to obtain a 3 mmol/l solution.
Specimen Collection
Blood (9 volumes) is mixed with 0.1 mol/l sodium citrate (1 volume) and centrifuged at 2000 x g for 20 min at 20-25°C. Separate plasma carefully from blood cells. Perform the analysis within 24 hours when plasma is stored at 2-25°C. Alternatively, freeze aliquots < 1ml at -20°C or below.
Perform the analysis of frozen samples within two months when stored at -20°C or within one year when stored at -70°C or below. No significant loss of prothrombin activity occurs upon freezing once, provided freezing is made in small aliquots (< 1 ml) and thawing is performed in a water bath or in an electric heater at 25-37°C.
Sample and Standard Dilutions
Standards
Calibrated normal plasma is diluted 1:23 – 1:160 to provide standard concentrations of 25-175%. The following table provides a suggestion of standard dilutions.
Standard Dilution Prothrombin Activity
| Standard Dilution | Prothrombin Activity |
|---|---|
| 1:23 | 175% |
| 1:29 | 138% |
| 1:40 | 100% |
| 1:80 | 50% |
| 1:160 | 25% |
Samples
Plasma samples are diluted 1:40 in Tris BSA Buffer working solution for application on microplate and diluted 1:80 for application on ACL (see below).
Microplate Assay Procedure
| Standard/Sample dilution | 50 µl |
| Incubate at 37ºC | 2-4 min |
| Ecarin or Echis Carinatus (37ºC) | 50 µl |
| Incubate at 37ºC | 3 min |
| Substrate (37ºC) | 50 µl |
| Read kinetically or incubate at 37ºC | 2 min |
| Acetic acid, 20% | 50 µl |
Determine the absorbance difference A405nm-490 nm for the standard dilution and the samples. Draw a standard curve from the absorbances obtained for the standard dilutions. Read the prothrombin activity for the samples from the standard curve.
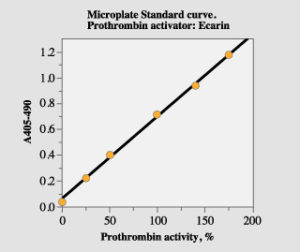
Figure 1. Standard curve with the microplate method.
Application on ACL
Use the plasminogen channel program. Prepare a standard dilution 1:40, which corresponds to a nominal prothrombin activity of 100% (see above regarding calibration). Standard dilutions corresponding to 25% and 50% are then automatically prepared by the instrument. In order to allow determination of prothrombin activity up to 200%, sample plasma should be diluted 1:80 and the obtained result should be multiplied with two.
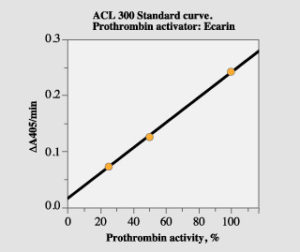
Figure 2. Standard curve with the ACL method.
Expected values (13)
The normal range is 75 – 130% (mean 102% 2 SD) as determined from analysis in microplate and on the ACL 300 of 101 healthy individuals (49 men and 52 women; age range 20 – 68 years). Analysis of plasma from 42 carriers of the G20210A mutation, who were not on oral anticoagulant treatment at the time of blood sampling, resulted in an activity range of 94 – 164% (mean 128% 2SD).
Interference and Limitations
No influence in the assay is obtained from variation of antithrombin activity in the range 50 – 150% of normal. Since meizothrombin is formed and measured, no influence in the assay is obtained from heparin levels < 1 IU/ml plasma. Since Ecarin also activates decarboxyprothrombin, which is produced during oral anticoagulant therapy with anti-vitamin K drugs, plasma from patients undergoing such treatment should not be analyzed with this method.
Repeatability
The imprecision, expressed as CV, within and between series (7 series, 5 replicates in each series) is < 4% at 50% and 100% prothrombin activity.
Bibliography
- Lane DA, Mannucci PM, Bauer KA, Bertina RM, Bochkov NP, Boulyjenkov V, Chandy M, Dahlbäck B, Ginter EK, Miletich Jp, Roosendaal FR, Selingsohn U. Inherited Thrombophilia: part 1. Thromb Haemost 76, 651-662 (1996).
- Poort SR, Roosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 88, 3698-3703 (1996).
- Hillarp A, Zöller B, Svensson PJ, Dahlbäck B. The 20210 allele of the prothrombin gene is a common risk factor among Swedish out-patients with verified deep venous thrombosis. Thromb Haemost 78, 990-992 (1987).
- Cumming AM, Keeney S, Salden A, Bhavnani M, Shwe RH, Hay CRM. The prothrombin gene G20210A variant: prevalence in a UK anticoagulant clinic population. Br J Haematol 98, 353-355 (1997).
- Brown K, Luddington R, Williamson D, Baker P, Baglin T. Risk of venous thromboembolism associated with a G to A transition at position 20210 in the 3’-untranslated region of the prothrombin gene. Br J Haematol 98, 907-909 (1997).
- Makris M, Preston FE, Beuchamp NJ, Hampton KK, Daly ME, Cooper P, Bayliss P, Peake IR. Co-inheritance of the 20210A allele of the prothrombin gene increases the thrombotic risk in subjects with familial thrombophilia. Thromb Haemost 78, Suppl., 165 (1997).
- Ferraresi P, Marchetti G, Legnani C, Cavallari E, Castoldi E, Mascoli F, Ardissino D, Palareti G, Bernardi F. The heterozygous 20210G/A prothrombin genotype is associated with early venous thrombosis in inherited thrombophilias and is not increased in frequency in artery disease. Arterioscl Thromb Vasc Biol 17, 2418-2422 (1997).
- Kapur RK, Mills LA, Spitzer SG, Hultin MB. A prothrombin gene mutation is significantly associated with venous thrombosis. Arterioscl Thromb Vasc Biol 17, 2875-2879 (1997).
- Howard TE, Marusa M, Channel C, Duncan A. A patient homozygous for a mutation in the prothrombin gene 3’-untranslated region associated with massive thrombosis. Blood Coag Fibrinol 8, 316-319 (1997).
- Martinelli I, Sacchi E, Landi G, Taioli E, Duca F, Mannucci PM. High risk of cerebral-vein thrombosis carriers of a prothrombin-gene mutation and in users oral cotraceptives. New Engl J Med 338, 1793-1797 (1998).
- Koster T, Blann AD, Briët E, Vanenbroucke JP, Roosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. The Lancet 345, 151-155 (1995).
- D’Onnel J, Tuddenham EGD, Manning R, Kemball-Cook Johnson D, Laffan M. High prevalence of elevated factor VIII levels in patients referred for thrombophilia screening: role of increased synthesis and relationship to the acute phase reaction. Thromb Haemost 77, 825-828 (1997).
- Rosén S, Andersson M, Ghosh R. A new chromogenic prothormbin method providing accurate determination of elevated prothormbin activity in plasma samples. ISTH 1999, Abstract 269.
