Determination of hirudin levels in plasma with the Chromogenic Substrate S-2366™
Background
Hirudin is a protein originating from the medical leech (1,2). It is the most potent thrombin inhibitor with a dissociation constant below 10 -12 mol/l. Due to the production of hirudin through recombinant technology, this protein is now readily available in abundant amounts and hence it has become an interesting candidate as a new antithrombotic agent (3-6). The therapeutic range of hirudin is roughly 0.5 – 2.5 µg/ml. Hirudin may be determined by ELISA methods, by clotting methods (APTT, thrombin time or Ecarin clotting time (ECT)) or by chromogenic methods (7,8). The latter is insensitive to oral anticoagulants (9) and is suitable for automation. The ECT method is the most competitive of the clotting methods being essentially insensitive towards heparin. However it shows some sensitivity to changes in plasma concentrations of prothrombin and fibrinogen (9, 10).
The chromogenic methods described here may be used for the monitoring of hirudin levels in plasma samples. The same principle may be used for the potency assessment of hirudin preparations. In this case the potency of hirudin is expressed in thrombin inhibitory units (TIU) (11) and the international standard for thrombin must be used.
Measurement principle
Thrombin is added in excess to the sample. Thrombin activity is neutralized in proportion to the amount of hirudin contained in the sample, and the remaining amount hydrolyses the chromogenic substrate S-2366.
The pNA released upon hydrolysis of the substrate is then monitored photometrically at 405 nm.
Thrombin (excess) + Hirudin → Thrombin Hirudin + Thrombin (residual)
Thrombin
S-2366 → pNA + Peptide
Reagents
- Chromogenic Substrate S-2366, 25 mg, Art. No. 82 10 90
Reconstitute with 20 ml sterile water. - Human Thrombin, 10 NIH-U (Sigma, T-9135)
Reconstitute with 7.1 ml sterile water to obtain a solution 1.4 NIH-U/ml. The solution is stable for one week at 2-8°C - Polybrene ® (Sigma, H-9268)
Dissolve the substance with water to obtain 1 mg/ml. - Tris EDTA Buffer, Art. No. B823366
10 ml stock solution Buffer containing 0.5 mol/l Tris pH 8.4, 1.5 mol/l NaCl, 70 mmol/l Disodium-EDTA. An opened vial is stable for 2 months at 2-8°C. Before use, dilute 10 ml of the stock solution with 88.7 ml sterile water. Add 1.26 ml polybrene solution. - Hirudin
Prepare a stock solution of 500 µg/ml hirudin.
Specimen collection
Blood (9 volumes) is mixed with 0.1 mol/l sodium citrate (1 vol) and centrifuged at 2000 x g for 20 minutes at 20-25°C. Separate plasma carefully from the blood cells.
Standard and Sample Dilution
Standards
Low range (0 – 2 µg/ml)
| Hirudin µg/ml | Predilution – Hirudin Stock µl | Predilution – Water µl | Final Dilution – Dil. Hirudin µl | Final Dilution – Plasma µl |
|---|---|---|---|---|
| 2.0 | 200 | 300 | 10 | 1000 |
| 1.5 | 150 | 350 | 10 | 1000 |
| 1.0 | 100 | 400 | 10 | 1000 |
| 0.5 | 50 | 450 | 10 | 1000 |
| 0.0 | – | – | – | 1000 |
High range (0 – 10 µg/ml)
| Hirudin µg/ml | Hirudin Stock µl | Plasma µl |
|---|---|---|
| 10.0 | 20 | 1000 |
| 7.5 | 15 | 1000 |
| 5.0 | 10 | 1000 |
| 2.5 | 5 | 1000 |
| 0.0 | – | 1000 |
Samples and Standards
| Dilution | Low dose range | High dose range |
|---|---|---|
| Sample / Standard | 50 µl | 25 µl |
| Buffer | 800 µl | 2000 µl |
Microplate Assay Procedure
| Sample | Blank | |
|---|---|---|
| Samples/Standard | 50 µl | 50 µl |
| Incubate at 37°C | 3-4 min | – |
| Thrombin (20-25°C) | 50 µl | – |
| Incubate at 37°C | 2 min | – |
| Substrate | 50 µl | – |
| Incubate at 37°C | 2 min | – |
| Acetic acid, 20% | 50 µl | 50 µl |
| Water | – | 100 µl |
Read the absorbance at 405 nm, using a reference wavelength of 490 nm. The color is stable for at least 4 hours. Subtract the absorbance for the blank from the absorbance of the corresponding standards and test plasma sample. Plot the corrected absorbances for the standards against hirudin concentrations in a lin-lin graph and draw the standard curve from linear regression.
The concentration of hirudin in the test sample is calculated from the standard curve.
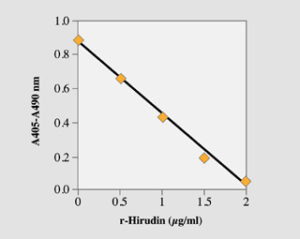
Fig. 1. Low range standard curve with the microplate method. Recombinant hirudin was used.
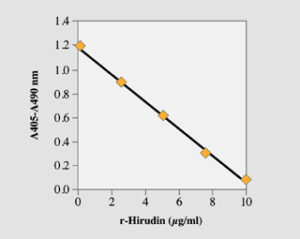
Fig. 2. High range standard curve with the microplate method. Recombinant hirudin was used.
Bibliography
- Markwardt F. Untersuchungen über hirudin. Naturwissenschaften 42; 537-538 (1955).
- Markwardt F. Hirudin as an inhibitor of thrombin. Meth Enzymol 19; 924-931 (1970).
- Markwardt F, Hauptmann J, Nowak G, Klessen C, Walsmann P. Pharmacological studies on the antithrombotic action of hirudin in experimental animals. Thromb Haemost 47; 226-229 (1982).
- Walenga J, Pifarré R, Fareed J. Recombinant hirudin as an antithrombotic agent. Drugs of the Future 15; 267-280 (1990).
- Johnson PH, Sze P, Winant R, Payne PW, Lazar JB. Biochemistry and genetic engineering of hirudin. Semin Thromb Haemost 15; 302-315 (1989).
- Märki WE, Grossenbacher H, Grutter MG, Liersch MH, Meyhack B, Heim J. Recombinant hirudin: genetic engineering and structure analysis. Semin Thromb Haemost 17; 88-93 (1991).
- Lindhoff-Last E, Mosch G, Rabe FW, Schenk J, Breddin HK. Chromogenic substrate assay for automatic determination of high dose and low dose hirudin levels in plasma. GTH (Gesellschaft für Thrombose- und Hämostaseforschung), 1993.
- Lindhoff-Last E, Bauersachs R, Mosch G, Piechottka GP, Rabe FW, Ehrly AM. A chromogenic method for the determination of hirudin in plasma. GTH (Gesellschaft für Thrombose- und Hämostaseforschung), 1999.
- Lindhoff-Last E, Piechottka GP, Rabe FW, Bauersachs R. Hirudyn determination in plasma can be strongly influenced by the prothrombin level. Thromb Res 100 (1), 55-60 (2000).
- Nowak G. Monitoring of the action of antithrombin agents by Ecarin clotting time. In: New Anticoagulants for the Cardiovascular Patient. Ed Pifarré. Hanley and Belfus Inc. Philadelphia PA, USA (1997).
- Longstaff C, Wong M, Gaffney PJ. An international collaborative study to investigate standardisation of hirudin potency. Thromb Haemost 69; 430-435 (1993).
